Details of the Drug
General Information of Drug (ID: DMZBNH0)
| Drug Name |
NISOXETINE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Nisoxetine; 3-(2-Methoxyphenoxy)-N-methyl-3-phenylpropylamine; Compound 89218; 3-(2-methoxyphenoxy)-N-methyl-3-phenylpropan-1-amine; Nisoxetine [USAN:INN]; 3-(o-Methoxyphenoxy)-N-methyl-3-phenylpropylamine; N-Methyl-gamma-(2-methylphenoxy)phenylpropanolamine; Lilly 94939; 57226-61-6; DL-N-Methyl-3-(o-methoxyphenoxy)-N-methyl-3-phenylpropylamine; CHEMBL295467; 53179-07-0; CHEBI:73410; ITJNARMNRKSWTA-UHFFFAOYSA-N; 57754-86-6; NCGC00015715-04; Nisoxetine Inhibitor; DSSTox_RID_80724; DSSTox_CID_25175; DSSTox_GSID_45175
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
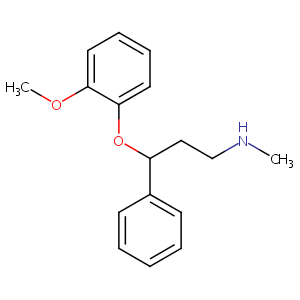 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 271.35 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
